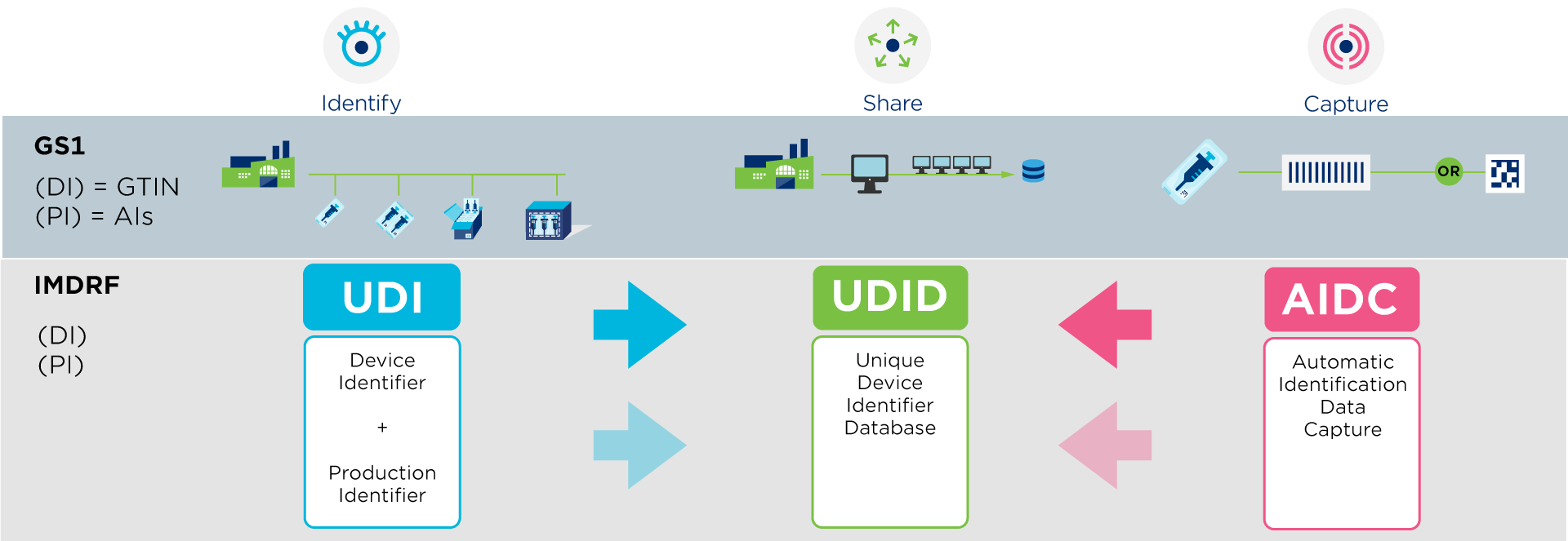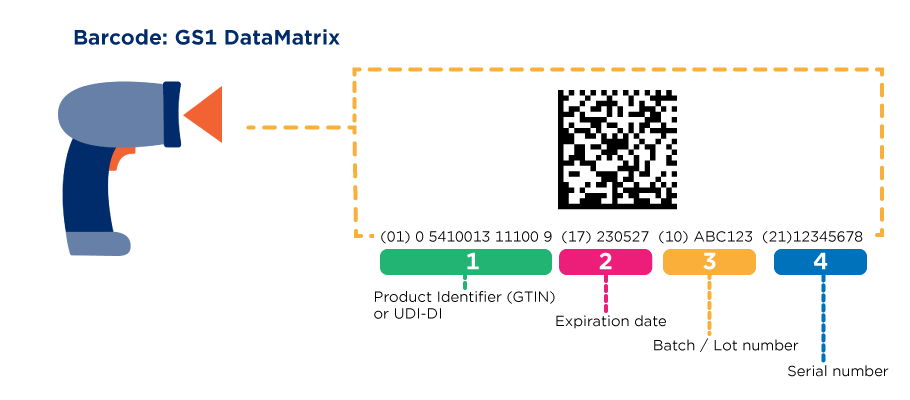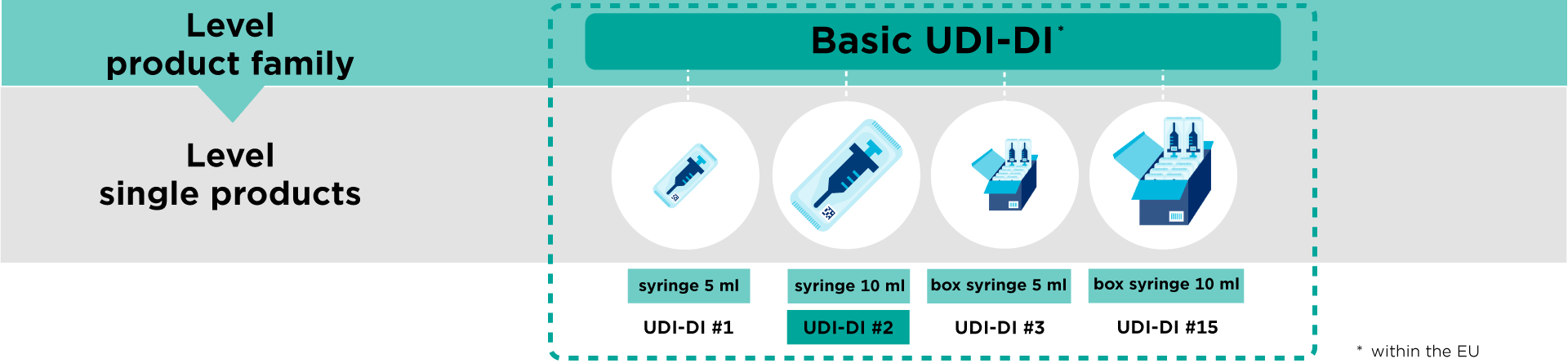
A UDI (Unique Device Identifier) is a code that is unique in the world for identifying medical devices. The code consists of two parts with numeric or alphanumeric characters: a fixed part, the Device Identifier (DI), and a variable part, the Production Identifier (PI).
GS1 is an accredited UDI-issuing organisation that helps you comply with international regulations, such as EU MDR & IVDR and US FDA, that require suppliers to assign a UDI to their products.
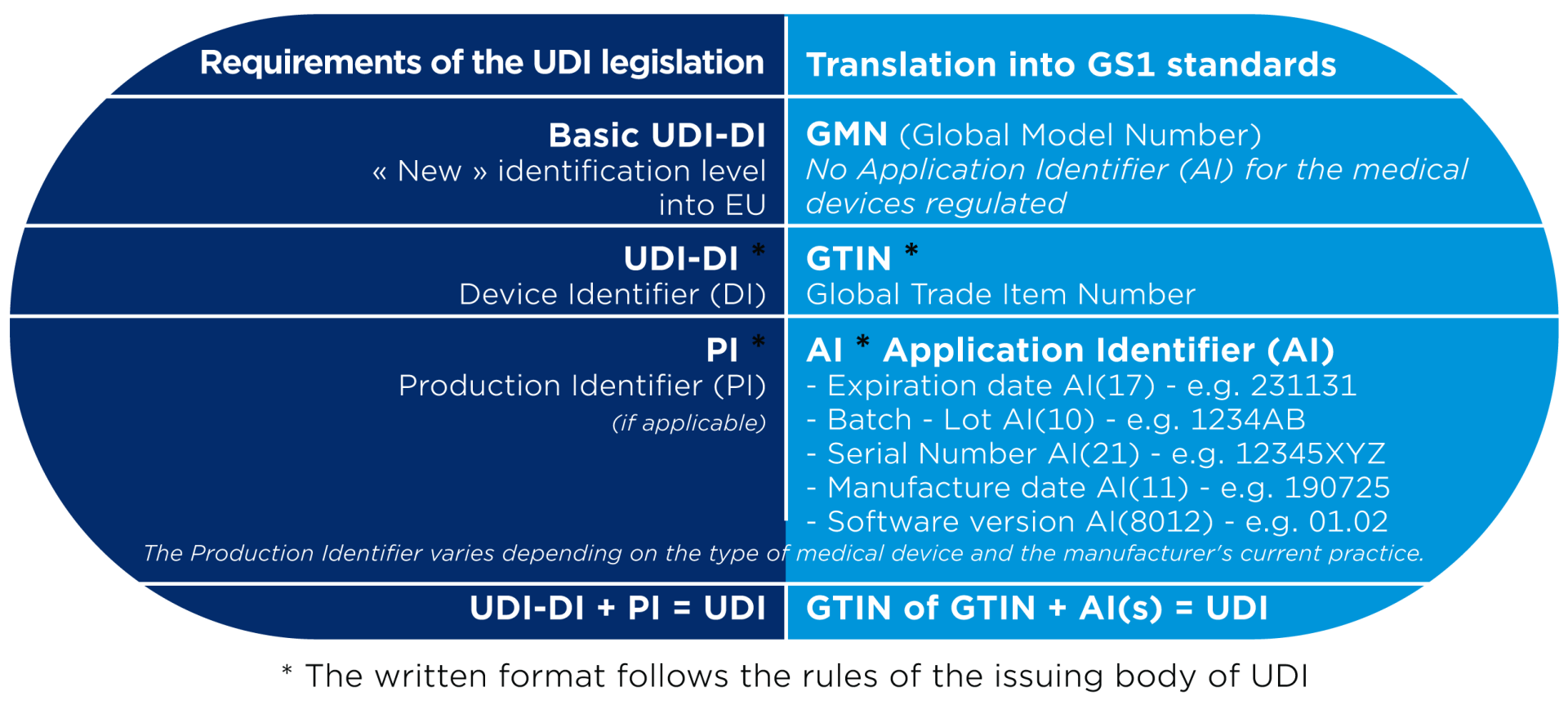
As a supplier, you are required to register your data (= UDI-DI) in a regulated government database.
For America (FDA), it is GUDID and you can publish your UDI-DI from My Product Manager.
For Europe, it is EUDAMED. You can publish your data via My Basic UDI-DI Manager. Send an e-mail to healthcare@gs1belu.org, should you be interested in this.