
8 steps to identify a medical device and create its UDI-DI easily

Select your main UDI contact person
Determine who is responsible for managing, creating and publishing the UDI-DI and Basic UDI-DI in your organisation.

Estimate the number of product codes
Think carefully about how many variations and packaging there will be. You must purchase a code pack depending on the number of UDI-DI you will need. Better too much than too little!
Order your product package and become a GS1 member
If you are a GS1 member, you can use our standards, barcodes and the My Product Manager platform. In this, you can create product codes / GTIN (UDI-DI) for your products or any identification key (GLN, GSRN, GRAI,...) you need.

Follow a training and create an UDI-ID
To be aware of what should and may be done, GS1 offers a number of useful training courses.
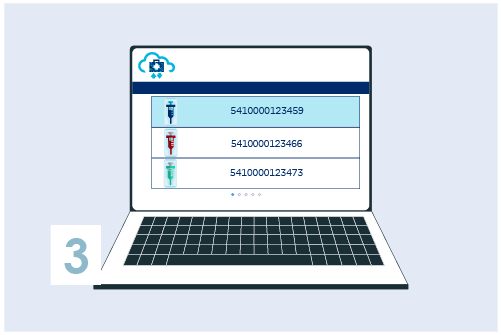
Register your GTIN (UDI-DI) in My Product Manager
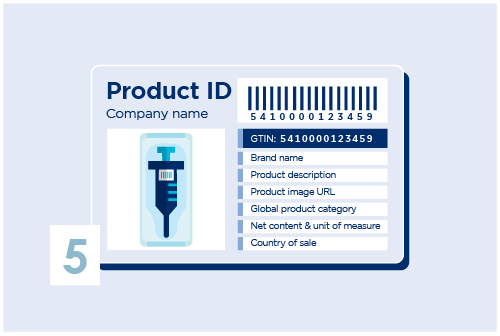
Assign UDI-DIs to each version or model of the medical device as well as to all different packaging levels. Create the UDI-DI in My Product Manager with basic information.

Choose the right barcode and/or identification key
Add a barcode on your products. Choose a GS1 Datamatrix or a GS1-128. Other useful standards are also provided by GS1, use the right identification keys that suits your needs.
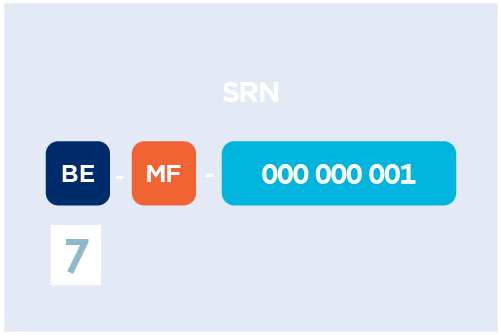
Apply for a SRN
Apply for a SRN (Single Registration Number) to access EUDAMED.
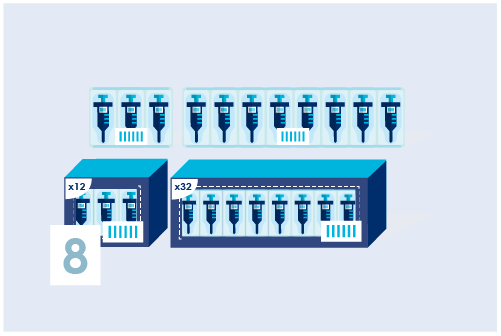
Assign a Basic UDI-DI (only for Europe)
Identify product models and/or product families with a Basic UDI-DI. This can be done in 2 ways:
- directly in Eudamed via the Eudamed page or
- by using My Basic UDI-DI Manager
Exchanging product data
Share your product data with hospitals or other partners in Belgium and abroad. Thanks to My Product Manager, the central place to publish product data, you can extand your data using the ECHO data model. In addition, you can register your Basic UDI-DI in Eudamed.
Morer info about My Product Manager
Request access to My Product Manager
Register your UDI-DI in Eudamed
Register your UDI-DI in Eudamed via My Basic UDI-DI Manager. My Basic UDI-DI Manager is a part of My Product Manager.
You create your Basic UDI-DIs and insert the necessary information as well as the link to UDI-DI that you have already created in My Product Manager. When all data are ready and complete you can forward them to EUDAMED.
