When does my trade item need another GTIN?
Identification of trade items in the Healthcare sector
Identifying trade items with unique numbers can be a real challenge. While it is a crucial step in ensuring traceability throughout supply chains for any given sector, the stakes in the HC sector are even higher, since patients’ lives can be heavily impacted by medication or medical supplies. Imagine for a second the risks that could arise if there was an identification error on the medicines given at a pharmacy or the medical devices used in a hospital for an operation.
GS1 Healthcare has developed and promoted global industry standards to prevent medical errors, combat counterfeit products and improve supply chain efficiency throughout the global healthcare industry.
When it comes to identification, the sector has since long decided to use Global Trade Item Numbers (GTINs). Using such GTINs for the sector can be combined with additional elements to guarantee even greater security and traceability. This is why it is common to see, next to the GTINs and barcodes, additional information such as a batch number or a unique serial number or an expiry date. It is important that all of these data are standardized throughout the supply chain. The integrity of these identification keys all along the item's lifetime is vital to maintaining uniqueness for manufacturers, wholesalers, distributors, hospitals, regulatory entities and other supply chain stakeholders.
But what happens if you have to change one aspect, characteristic, variant or formulation of a trade item?

GTIN Allocation Rules
This is where the GTIN Allocation Rules come in. Certain changes to a trade item, or its packaging, may require the allocation of a new GTIN, while others don’t.
The Healthcare GTIN Allocation Rules help companies make consistent decisions about how to manage the unique identification of trade items, therefore contributing to improved supply chain efficiency and patient safety. Business partners rely on unique identification of trade items for unambiguous data exchange in order to maintain operational efficiency. It also ensures the smooth operation of global supply chains, while supporting the implementation of various regulatory requirements worldwide.
Hence, it is not only essential to allocate a new GTIN to a trade item when required; but also to communicate these changes to trading partners, ensuring that the right trade item is provided, or that the item is used as intended.
A concrete example
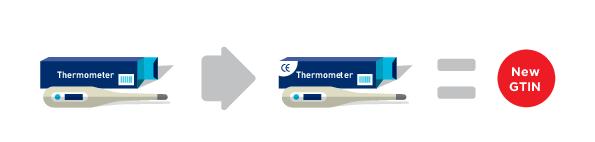
A change to packaging to add a new or remove an existing certification mark (e.g., European Certification Mark CE), that has significance to regulatory bodies, trading partners or to the end consumer, requires assignment of a new GTIN.
For the purpose of interpretation of this rule, a certification mark is a symbol, logo or wording on a product that declares a product has met specific criteria and standards in formulation, harvesting, processing or manufacturing (e.g., European Certification Mark) and that can be externally verified by a certification authority or agency which can be either a public or private authority.
Interactive Decision Support Tool
To further assist stakeholders in understanding when to change a GTIN used within open supply chains, GS1 has created the GS1 Healthcare GTIN Allocation Rules Decision Support Tool. Discover more about the Decision Support Tool



