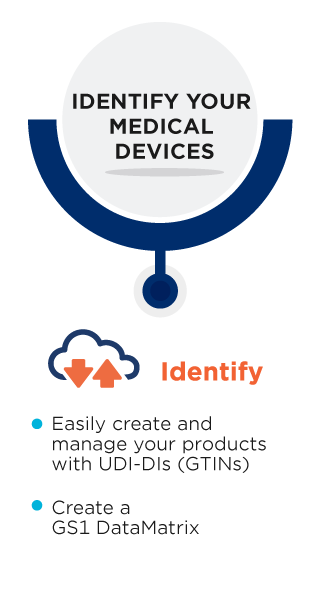
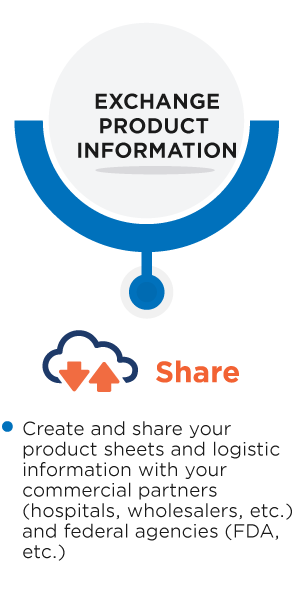
The Unique Device Identifier (UDI) is a medical device identification system used by various regulators worldwide.
Thanks to the UDI, medical devices are uniquely identified, which increases patient safety and efficiency. This allows better management of medical device recalls and will reduce medical errors.

Recognised worldwide
Efficient and effective processes

Compliant with the legislation
What would you like to do?